Phosphorus is one of the main “ingredients” for healthy plant growth. Plants get phosphorus from the soil. And much of this phosphorus is applied to farm fields in the form of fertilizers.
The major current source of phosphorus, which comes from rock, is running out. Plus, it must be mined, and then chemically converted and transported long distances. This costs money and uses valuable resources.
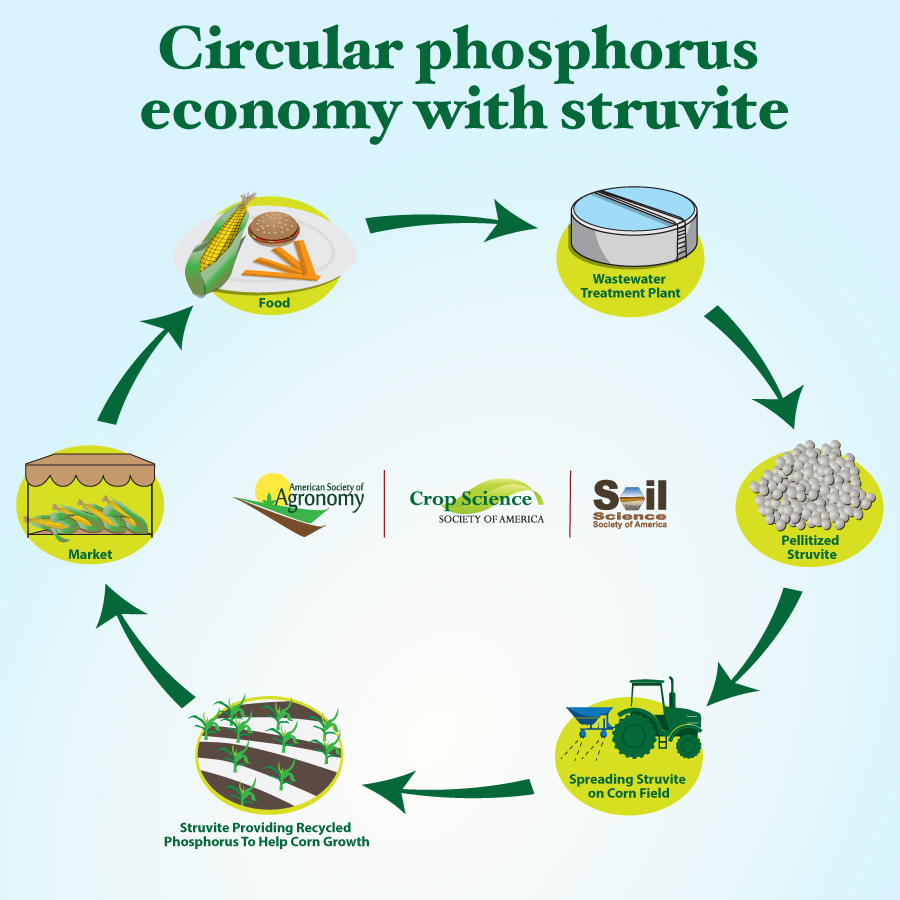
Research is looking at alternative ways to retrieve phosphorus, such as in the form of struvite. Struvite is a chemical compound that contains not only phosphorus, but magnesium and other elements like nitrogen. All of these are important nutrients for crops. Struvite has shown promise for use in agriculture as an alternative fertilizer-phosphorus source. Both greenhouse and row-crop studies suggest that struvite is a viable fertilizer compound, compared to traditional fertilizers.
How is struvite recovered from wastewater?
You might be familiar with the term precipitation. It’s what happens when you make rock candy – you dissolve enough sugar into hot water and larger crystals of sugar form as the water cools.
Wastewater treatment plants work to purify water so that it can be reused. They fall under strict standards from the Environmental Protection Agency. Struvite can precipitate from wastewater in similar ways to rock candy formation. Scientists have found that it is not only possible to pull struvite from wastewater, but that it can be done on a large scale.
Researchers have developed new technologies to precipitate struvite at strategic locations in wastewater treatment plants. They’ve also found ways to precipitate struvite from other liquid waste streams, including municipal landfills and animal and industrial wastewaters. All these processes remove excess phosphorus and nitrogen and make them available for reuse.
One way to cause struvite to precipitate from wastewater is to add other chemicals in the wastewater stream. This chemical conversion causes struvite crystals to form, which can eventually be turned into small pellets with additional processing.
More recently, researchers have developed ways to use electrochemically precipitation to generate struvite. By applying an electrical current to a phosphorus- and nitrogen-containing solution, struvite crystals form on the magnesium electrode – or may even fall to the bottom of the container. The sinking crystals can be collected out of the solution. The struvite on the magnesium electrode can also be dried and scraped off for collection.
Both chemically and electrochemically precipitated struvite can be used as a recycled fertilizer product. Our research shows struvite can be an effective alternative to traditional fertilizer phosphorus additions. This creates a circular “nutrient economy” – where the struvite is used as fertilizer, which is then eaten as food, recovered in wastewater, and then reused. It not only helps the environment by keeping phosphorus and nitrogen out of water bodies, but conserves phosphate rock mineral resources. In addition, it may be possible to use struvite that is made regionally, reducing the transportation costs and greenhouse gas emissions from transportation.
Does precipitating struvite have other benefits?
Precipitating phosphorus and nitrogen from wastewater is also helpful for aquatic ecosystems. Both excess phosphorus and nitrogen can cause eutrophication, which can eventually create dead zones. This is a major water quality issue in many places of the world, including Lake Erie and the Gulf of Mexico. Algae need nutrients to grow, and phosphorus and nitrogen are part of those nutrients. But when there is too much of either nutrient, particularly phosphorous, algae production increases more than usual. The algae cover the water’s surface. This is followed by oxygen-consuming decomposition that can create a zone of oxygen depletion. This can kill other plants and even fish.
The removal of excess nutrients from wastewaters, particularly phosphorus and nitrogen, improves the quality of the water discharged back into the environment.
Struvite’s slow release may benefit the environment
Struvite behaves differently in various pH conditions. Struvite dissolves very slowly under neutral to basic conditions (pH 7 or greater). Soil becomes somewhat acidified (pH less than7) around plant roots. And under acidic soil conditions, struvite’s ability to dissolve increases. As struvite slowly dissolves near plant roots, the phosphorus, nitrogen, and magnesium become available for plant uptake when the plants need the nutrients. This is a bit like slow-release pain medicine. As a result, struvite has slow-release properties in soil when used as an agricultural phosphorus source. Struvite has been applied to fields either by surface application or by mixing into the soil.
When other commonly used, commercially available inorganic fertilizer sources are used, the phosphorus typically quickly binds to the soil. This makes the phosphorus unavailable for plant uptake. Because of struvite’s slow-release behavior, it may reduce the amount of phosphorus escaping into waterways and be helpful to the environment.
2022 marks the 50-year anniversary of the Clean Water Act. This Act aims to restore and maintain the physical, chemical, and biological integrity of the nation’s water resources. Struvite may play a future role in accomplishing the objectives of the Clean Water Act. Struvite may also play an integral role in supporting the sustainability of agriculture and food production with recycled nutrient fertilizer sources.
Research about struvite and its uses will help keep people fed and protect the environment, just as the Clean Water Act envisioned.
Authors Kristofor R. Brye and Lauren Greenlee recently.